Comparative characteristics of competitive and non-competitive inhibition. Enzyme inhibitors. Feedback type regulation
Distinguish between reversible and irreversible inhibition. If the inhibitor causes persistent changes in the spatial tertiary structure of the enzyme molecule or modification of the functional groups of the enzyme, then this type of inhibition is called irreversible. More often, however, there is a reversible inhibition, amenable to quantitative study on the basis of the Michaelis-Menten equation. Reversible inhibition, in turn, is divided into competitive and noncompetitive, depending on whether it is possible or not possible to overcome the inhibition of the enzymatic reaction by increasing the substrate concentration.
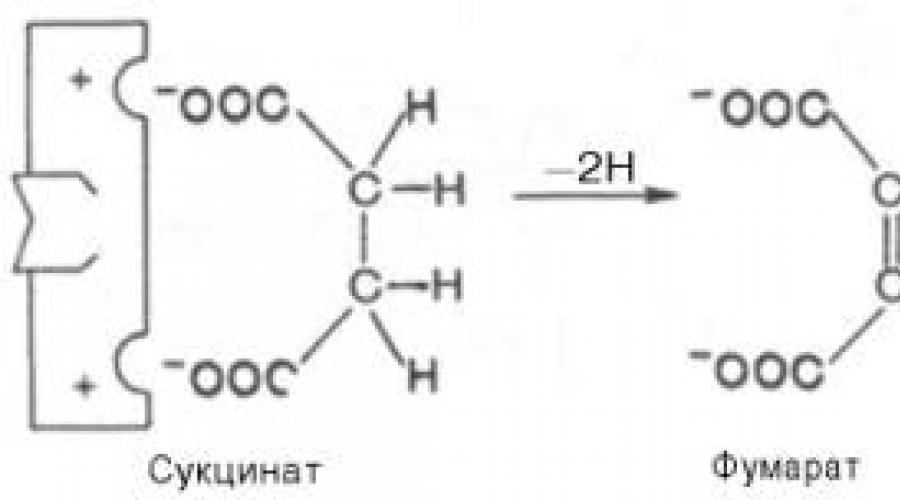
Competitive inhibition can be caused by substances that have a structure similar to that of the substrate, but slightly different from the structure of the true substrate. This inhibition is based on the binding of the inhibitor to the substrate-binding (active) center. A classic example of this type of inhibition is the inhibition of succinate dehydrogenase (SDH) by malonic acid. This enzyme catalyzes oxidation by dehydrogenation succinic acid(succinate) to fumaric:
If malonate (inhibitor) is added to the medium, then, as a result of its structural similarity with the true substrate succinate (the presence of two of the same ionized carboxyl groups), it will interact with the active center to form an enzyme-inhibitor complex, but this completely excludes the transfer of a hydrogen atom from malonate ... The structures of the substrate (succinate) and inhibitor (malonate) are still somewhat different. Therefore, they compete for binding with the active site, and the degree of inhibition will be determined by the ratio of the concentrations of malonate and succinate, and not by the absolute concentration of the inhibitor. Thus, the inhibitor can reversibly bind to the sphere, forming an enzyme-inhibitor complex. This type of inhibition is sometimes referred to as metabolic antagonism inhibition (Figure 4.20).
V general form the reaction of interaction of an inhibitor with an enzyme can be represented by the following equation:
The resulting complex, called the enzyme-inhibitory complex EI, in contrast to the enzyme-substrate complex ES, does not decompose with the formation of reaction products. The dissociation constant of the EI complex, or the inhibitory constant К i, can, following the Michaelis – Men-ten theory, be defined as the ratio of the constants of the reverse and direct reactions:
The competitive inhibition method has found wide application in medical practice. It is known, for example, that for the treatment of some infectious diseases caused by bacteria, sulfa drugs are used. It turned out that these drugs have a structural similarity to sparaaminobenzoic acid, which the bacterial cell uses to synthesize folic acid, which is an integral part of
Rice. 4.20. The action of a competitive inhibitor (scheme according to V.L.Kretovich). E - enzyme; S - substrate; Р 1 and Р 2 - reaction products; I is an inhibitor.
enzymes of bacteria. Due to this structural similarity, sulfonamide blocks the action of the enzyme by displacing para-aminobenzoic acid from the complex with the enzyme synthesizing folic acid, which leads to inhibition of bacterial growth.
Noncompetitive inhibition is caused by substances that have no structural similarity to substrates and often bind not to the active site, but to another location in the enzyme molecule. The degree of inhibition in many cases is determined by the duration of the action of the inhibitor on the enzyme. With this type of inhibition, due to the formation of a stable covalent bond the enzyme often undergoes complete inactivation, and then the inhibition becomes irreversible. An example of irreversible inhibition is the action of iodoacetate, DPP, as well as diethyl-n-nitrophenyl phosphate and hydrocyanic acid salts. This action consists in binding and disabling functional groups or metal ions and an enzyme molecule.
Table of contents of the subject "Inhibition. Cell.":
In this case, a substance that is close in its structure to a common enzyme substrate, binds to the active center of the enzyme, but cannot react with it. Being here, it blocks access to the active center for any molecule of the real substrate.
Since in this case the inhibitor and the substrate compete for a place on active center of the enzyme This form of inhibition is called competitive inhibition. For competitive inhibition, it is characteristic that if the concentration of the substrate increases, then the reaction rate increases, i.e., this inhibition is reversible.
The figure illustrates one of examples of competitive inhibition.
Competitive inhibition phenomenon helps to understand the mechanism of action of some drugs, in particular sulfonamides. The goal of chemotherapy is to destroy the causative agent of the disease with the help of certain chemicals without damaging the tissues of the host organism. The first such drugs were sulfonamides, the antibacterial effect of which was discovered in the 30s of the XX century. During the Second World War, they were widely used to fight wound infections. Sulfonamides are chemically close to para-aminobenzoic acid (PABA), a necessary growth factor for many pathogenic bacteria... PABA is required by bacteria for the synthesis of folic acid, which serves as one of the cofactors of enzymes in them. Sulfonamides inhibit one of the enzymes involved in the synthesis of folic acid from PABA.
Animal cells insensitive to sulfonamides, although they require folic acid for some reactions. This is explained by the fact that they use already formed folic acid; they do not have a metabolic pathway that would ensure its synthesis.
Noncompetitive reversible inhibition
Inhibitors of this type are not related in their structure to the substrate of this enzyme; In this case, it is not the active center of the enzyme that participates in the formation of a complex with the inhibitor, but some other part of its molecule. This does not interfere with the binding of the substrate to the enzyme, but makes catalysis impossible.
With the rise inhibitor concentration the reaction rate is getting lower and lower. By the moment of saturation with the inhibitor, it turns out to be practically zero. In contrast to competitive inhibition, in this case, an increase in the substrate concentration does not affect the reaction rate.
Inhibition
Is the inhibition of enzyme activity. In this case, the denaturation of the enzymes does not occur.
Inhibitor - substance causing specific decreased activity enzyme. Inorganic acids and heavy metals are not inhibitors, but are inactivators, as they reduce the activity of any enzymes, i.e. act nonspecifically Denaturing agents are not also referred to as inhibitors.
Inhibitors: ions or small molecules that form part of the enzymatic regulatory system, as well as pharmacological drugs.
by the strength of binding of the enzyme with the inhibitor, inhibition is reversible and irreversible.
in relation to the ratio of the inhibitor to the active site of the enzyme, inhibition is divided into competitive and uncompetitive.
Inhibition types
1. Reversible 2. Irreversible
A. COMPETITIVE A. SPECIFIC
B. NON-COMPETITIVE B. NON-SPECIFIC
Reversible inhibition. Most inhibitors act reversibly to form non-covalent bonds with the enzyme, and under certain conditions dissociate with the restoration of enzyme activity.
Competitive inhibition.An inhibitor is similar to an enzyme substrate in its structure and competes with the substrate for the active center ( sits on the active center of the enzyme), which leads to a decrease in the binding of the substrate to the enzyme and disruption of catalysis. This is the feature of competitive inhibition - the ability to enhance or weaken inhibition through a change in the concentration of the substrate.
For the competitive type of inhibition, the following equations are valid:
E + S ⇔ ES → E + P,
1. Competitive interaction ethanol and methanol for the active center alcohol dehydrogenase.
2. Inhibition succinate dehydrogenase with malonic acid, the structure of which is similar to the structure of the substrate of this enzyme - succinic acid (succinate).

Succinate + FAD ----------- Fumarate + FADN 2
3. Competitive inhibitors also include antimetabolites or pseudosubstrates, such as antibacterial agents. sulfonamides similar in structure to P-aminobenzoic acid, a component of folic acid. When treating with sulfonamides in a bacterial cell, the use of P-aminobenzoic acid for synthesis folic acid, which causes a therapeutic effect.

The similarity of the structure of sulfonamides and para-aminobenzoic acid, a component of vitamin B9

Effect of different substrate concentrations on the rate of the reaction catalyzed by enzymes 1 and2 (in the presence of an inhibitor): a) hyperbolic dependenceVfrom [S], b) direct dependence in inverse coordinates 1 /Vfrom 1 / [S] - Lineweaver-Burke.
Competitive inhibitors reduce the rate of chemical reaction. A competitive inhibitor increases the K m for a given substrate (decreases the substrate's affinity for the enzyme). This means that in the presence of a competitive inhibitor a high concentration of substrate is required to achieve 1/2 V max. An increase in the ratio of the concentration of the substrate and the inhibitor decreases the degree of inhibition. At significantly higher substrate concentrations, inhibition disappears completely., because the active centers of all enzyme molecules will be predominantly in a complex with the substrate.
Noncompetitive inhibition.Inhibitordoes not have structural similarity to the substrate andjoins not in the active center, and in another place of the molecule, simultaneously with the substrate. A triple complex is formed: substrate - enzyme - inhibitor. This leads to deformation of the active site and catalytic activity. For instance, hydrocyanic acid (cyanides) binds to heme iron of the respiratory chain enzymes and blocks cellular respiration.

Kinetic dependence of noncompetitive inhibition: characterized by a decrease in V max of the enzymatic reaction and a decrease in the affinity of the substrate for the enzyme, i.e. increase K m.

Noncompetitive inhibition in double reciprocal coordinates at different concentrations of the inhibitor (1 - [I] = 0; 2 - [I]> 0; 3 - [I]> [I] 2).
With noncompetitive inhibition, the Michaelis constant does not change, and the maximum reaction rate decreases in (1 + [ I]/K i) once. Therefore, in double reciprocal coordinates, the family of straight lines corresponding to different concentrations of the inhibitor intersect at one point on the abscissa. Irreversible inhibition is observed in the case of the formation of covalent stable bonds between the inhibitor molecule and the enzyme. Most often, the active center of the enzyme undergoes modification.As a result, the enzyme cannot perform a catalytic function.
Irreversible inhibitors include heavy metal ions, for example, mercury (Hg 2+), silver (Ag +), and arsenic (As 3+), which in low concentrations block sulfhydryl groups of the active site. In this case, the substrate cannot undergo chemical transformation (Fig. 2-26). In the presence of reactivators, the enzymatic function is restored. In high concentrations, heavy metal ions cause denaturation of the protein molecule of the enzyme, i.e. lead to complete inactivation of the enzyme.
Orenburg - 2010
1.1 Reversible inhibition
1.1.2 Noncompetitive inhibition
1.1.3 Uncompetitive inhibition
1.2 Irreversible inhibition
1.3 Allosteric inhibition
2. The new kind inhibition of enzymatic activity
3. The use of enzyme inhibitors
CONCLUSION
List of used literature
1. Inhibitors of enzymes. Types of inhibition of enzyme activity
It is known that the activity of enzymes can be relatively easily reduced by various actions. Such a decrease in the rate of enzymatic reactions is usually called inhibition of activity, or inhibition of enzymes.
Fig 1. Scheme of activation and inhibition of the enzyme action (according to Yu. B. Filippovich): a. - allosteric center of the enzyme; K - catalytic center; c - substrate center
Enzymes are proteins, respectively, their activity can be reduced or completely eliminated by actions leading to denaturation of proteins (heating, the action of concentrated acids, alkalis, salts of heavy metals, etc.) is not of particular interest for studying their mechanism. Much more important is the study of inhibition using substances that specifically and usually in small amounts interacting with enzymes - enzyme inhibitors. Deciphering the mechanisms of many biological processes, such as glycolysis, the Krebs cycle and others, became possible only as a result of the use of specific inhibitors of various enzymes (N.E. Kucherenko, Yu.D. Babenyuk et al., 1988).
Some enzyme inhibitors are effective medicinal substances for the body of animals and humans, while others are deadly poisons (V.P. Komov, V.N. Shvedova, 2004).
Inhibitors interact with the active centers of the enzyme molecule, inactivating the functional groups of proteins. They can interact with metals that are part of enzyme molecules and enzyme-substrate complexes, inactivating them. High concentrations of inhibitors destroy the quaternary, tertiary and secondary structures of the enzyme molecule, causing its denaturation (A.I. Kononsky, 1992).
Recently, antienzymes (antienzymes, or antizymes) have been discovered, which are proteins that act as inhibitors of enzymes. Such substances include, for example, the trypsin inhibitor found in soybeans and serum antitrypsin. The anti-enzyme ornithine decarboxylase was recently discovered in animal liver. Antizymes, most likely, form hard-to-dissociate complexes with the corresponding enzymes, excluding them from chemical reactions. Sometimes the inhibitor is constituent component an enzyme precursor, or is part of complex enzyme complexes. However, it has not yet been clarified whether such antienzymes are true inhibitors or regulatory subunits.
If the inhibitor causes persistent changes in the spatial tertiary structure of the enzyme molecule or modification of the functional groups of the enzyme, then this type of inhibition is called irreversible. More often, however, there is a reversible inhibition, amenable to quantitative study on the basis of the Michaelis-Menten equation. Reversible inhibition, in turn, is divided into competitive and noncompetitive
In practice, many inhibitors do not exhibit the properties that are characteristic of purely competitive or purely noncompetitive inhibition. Another way to classify inhibitors is based on the nature of their binding site. Some of them bind to the enzyme in the same place as the substrate (in the catalytic center), while others - at a considerable distance from the active center (in the allosteric center) (R. Murray, D. Grenner et al., 1993).
1.1 Reversible inhibition
There are three types of reversible inhibition of enzymes: competitive, non-competitive and uncompetitive, depending on whether it is possible or not possible to overcome the inhibition of the enzymatic reaction by increasing the concentration of the substrate.
1.1.1 Competitive inhibition
The competitive inhibitor competes with the substrate for binding to the active site, but unlike the substrate, the competitive inhibitor bound to the enzyme does not undergo enzymatic conversion. Distinctive feature competitive inhibition is that it can be eliminated or weakened simply by increasing the concentration of the substrate. For example, if the enzyme activity is suppressed by 50% at given concentrations of the substrate and competitive inhibitor, then we can reduce the degree of inhibition by increasing the concentration of the substrate.
In their three-dimensional structure, competitive inhibitors usually resemble the substrate of this enzyme. Due to this similarity, the competitive inhibitor manages to "trick" the enzyme and bind to it. Competitive inhibition can be quantified based on the Michaelis-Menten theory. Competitive inhibitor I simply reversibly binds to enzyme E, forming a complex with it
Competitive inhibition can be most easily recognized experimentally by determining the effect of the inhibitor concentration on the dependence of the initial reaction rate on the substrate concentration. To clarify the question of what type - competitive or non-competitive - reversible inhibition of the enzyme occurs, the method of double reciprocal values is used. From the graphs constructed in double inverse coordinates, it is also possible to determine the value of the dissociation constant of the enzyme inhibitor complex (see Fig. 1) (A. Leinger, 1985)
Competitive inhibition can be caused by substances that have a structure similar to that of the substrate, but slightly different from the structure of the true substrate. This inhibition is based on the binding of the inhibitor to the substrate-binding (active) center (see Fig. 2).

Rice. 2. General principle competitive inhibition (scheme according to V.L. Kretovich). E - enzyme; S - substrate; Р 1 and Р 2 - reaction products; I is an inhibitor.
An example is the effect of malonic acid on a reaction catalyzed by succinate dehydrogenase and associated with the conversion of succinic acid to fumaric acid. The addition of malonic acid to the reaction mixture reduces or completely stops the enzymatic reaction, since it is a competitive inhibitor of succinate dehydrogenase. The similarity of malonic acid to succinic acid is sufficient for the formation of a complex with the enzyme, but the decomposition of this complex does not occur. With an increase in the concentration of succinic acid, it displaces malonic acid from the complex, as a result, the activity of succinate dehydrogenase is restored.

Rice. 3. Competitive inhibition of the reaction of conversion of succinic acid into fumaric acid under the action of malonic acid.
The structures of the substrate (succinate) and inhibitor (malonate) are still somewhat different. Therefore, they compete for binding with the active site, and the degree of inhibition will be determined by the ratio of the concentrations of malonate and succinate, and not by the absolute concentration of the inhibitor. Thus, the inhibitor can reversibly bind to the enzyme to form an enzyme-inhibitor complex. This type of inhibition is sometimes referred to as metabolic antagonism inhibition (see Figure 3).
In general form, the reaction of interaction of an inhibitor with an enzyme can be represented by the following equation:

The resulting complex, called the enzyme-inhibitory complex EI, in contrast to the enzyme-substrate complex ES, does not decompose with the formation of reaction products.
Many medicinal substances inhibit human and animal enzymes in a competitive manner. For example, sulfa drugs are used to treat some infectious diseases caused by bacteria. It turned out that these drugs have a structural similarity to para-aminobenzoic acid, which the bacterial cell uses to synthesize folic acid, which is an integral part of bacterial enzymes. Due to this structural similarity, sulfanilamide blocks the action of the enzyme by displacing para-aminobenzoic acid from the complex with the enzyme that synthesizes folic acid, which leads to inhibition of bacterial growth.

The structure of peptidoglycan of the bacterial cell wall includes D-alanine, which is absent in the body of animals and humans. To synthesize the cell wall, bacteria use the enzyme alanine-racemase to convert animal L-alanine into the D-form. Alanine racemase is characteristic of bacteria and is not found in mammals. Therefore, it represents a good target for inhibition drugs... Substitution of fluorine for one of the protons of the methyl group gives fluoroalanine, to which alanine racemase binds, which leads to its inhibition.