Calculate the amount of heat required to heat the mixture. Presentation on the topic "Calculation of the amount of heat required to heat the body and allocated by it during its cooling"

Slide 2.
The purpose of the lesson:
determine the formula for calculating the amount of heat required to change the temperature of the body; analyze the formula; testing of practical skills in solving problems; continue to learn to analyze the terms of the task; analyze and evaluate the answer of classmates;
Slide 3.
Without heat there is no life. But too strong cold and heat destroys everything alive. All the bodies, even ice blocks, emit energy, but poorly heated bodies emit little energy, and this radiation is not perceived by the human eye. In the eighteenth century, many scientists believed that the heat was a special substance "heatborne", weightless "liquid" contained in bodies. Now we know. It is not so. Today we will talk about the warmth and thermal phenomena, as well as learn how to calculate the amount of heat required to heat the body and stand out when cooling it.
Slide 4.
Comprehensive test of knowledge
1. The energy of the movement and interaction of the particles from which the body consists is called internal energy. 2. The internal energy of the body can not be increased by performing work on it. 3. Energy transfer from a colder body to more hotly called thermal conductivity. 4. With thermal conductivity, the substance does not move from one end of the body to another. 5. Convection occurs in solid bodies. 6. The energy that the body gives or gets under heat transfer is called the amount of heat. 7. Radiation is the type of heat transfer. 8. Energy transfer from one body to another or from one part to another molecules or other particles are carried out. 9. Internal energy is measured in Newton. 10. The amount of heat required to heat the body depends on the kind of substance
Slide 5.
Answers to the task:
Λ‗‗Λ‗ΛΛΛ‗Λ
Slide 6.
What picture shows three methods of heat exchange: thermal conductivity, radiation and convection? A / W / used /
Slide 7.
By thermal conductivity through the bottom and walls, the inner energy of the flame goes into the internal energy of tourist chowder. By radiation - in the inner energy of the palms of tourist and his clothes. And by convection - into the inner energy of the air over the fire.
Slide 8.
Qualitative tasks
From the Russian fairy tale "Fox - sister and gray wolf". The wolf went to the river, lowered the tail in the hole and began to sentence: "Fish, fish, and small and great! Fish, fish and small and great! " Following him and Lisa appeared; It goes near the wolf yes, it hits: "Clear, clear in the sky of the stars! Murzni, Murzni Wolf Tail! ". Tail and example. What way left the heat of the wolf's tail? (Radiation).
Slide 9.
From the Altai Fairy Tale "Mornosttai and Hare". Silently thought his Duma wise bear. In front of him, the big bonfire crackled, over the fire on the iron tripel stood a gold boiler with seven bronze ears. This Bear's favorite boiler never cleaned: I was afraid that, together with mud, happiness leaves, and the gold boiler was always a hundred soot layers as velvet covered. Did it affect the heating of water that the boiler was covered with a "strata of soot"?
Yes, as the soot are porous, then water heating will occur slower
Slide 10.
Before tosing, a night butterfly pulls up with wings for quite a long time. Why?
The butterfly "heats up", like an athlete making a workout before the start. A mechanical work performed by it goes to an increase in internal energy.
Slide 11.
Focus "Failed Paper". The nail is tightly wrapped with paper and heat the alcohol flame. Paper does not burn. Why? Focus "Failed Paper". The nail is tightly wrapped with paper and heat the alcohol flame. Paper does not burn. Why?
Iron has a great thermal conductivity, so almost everything is transmitted to the nail, and the paper does not burn. Experimental task.
Slide 12.
Experimental task. Experience with a striped glass of a glass of thin glasses, from the inside strips of white and black paper of the same width. Outside to the glass, glue the plasticine on one height of the button one against each white and black stripes. I put a glass on the saucer and in it a candle strictly to the center. I light a candle. After some time, the buttons begin to disappear. Explain the results of experience. Answer: At first, those buttons that are glued against the black strips of paper will be disappeared, since the glass is more heated here, the black surfaces absorb the energy of the radiation falling on them than white.
In practice, they often use thermal calculations. For example, during the construction of buildings, it is necessary to take into account how much heat should give the building the entire heating system. You should also know how much heat will go into the surrounding space through windows, walls, doors.
We will show on the examples how you need to lead the simplest calculations.
So, you need to know how the amount of heat obtained when heated copper item. Its mass is 2 kg, and the temperature increased from 20 to 280 ° C. At first, in Table 1, we define the specific heat capacity of copper with M \u003d 400 J / kg ° C). This means that on the heating of the components from the copper weighing 1 kg per 1 ° C, it will take 400 J. To heat the copper part 2 kg weighing 1 ° C, it is necessary 2 times the amount of heat - 800 J. The temperature of the copper part must be increased not to 1 ° C, and 260 ° C, it means, it will take 260 times more heat, i.e. 800 J 260 \u003d 208,000 J.
If we designate a mass M, the difference between the final (T 2) and the initial (T 1) temperatures - T 2 - T 1 we obtain the formula for calculating the amount of heat:
Q \u003d Cm (T 2 - T 1).
Example 1.. In the iron boiler weighing 5 kg of pouring water weighing 10 kg. What amount of heat should be transferred to a water boiler to change their temperature from 10 to 100 ° C?
When solving the problem, you need to consider that both bodies - and the boiler, and water - will be warm together. The heat exchange occurs between them. Their temperatures can be considered the same, i.e. the temperature of the boiler and water varies 100 ° C - 10 ° C \u003d 90 ° C. But the amount of heat obtained by the boiler and water will not be the same. After all, their masses and specific heat are different.
Water heating in the bowler
Example 2.. Water was mixed with a mass of 0.8 kg, having a temperature of 25 ° C, and water at a temperature of 100 ° with a mass of 0.2 kg. The temperature of the resulting mixture was measured, and it turned out to be equal to 40 ° C. Calculate how the amount of warmth gave hot water when cooled and got cold water when heated. Compare these amounts of warmth.
We write the condition of the task and solve it.

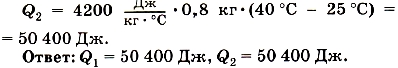
We see that the amount of warmth, given by hot water, and the amount of heat obtained cold waterare equal to each other. This is not a random result. Experience shows that if heat exchange occurs between the bodies, the internal energy of all heated bodies increases as much as the internal energy of cooling bodies decreases.
When conducting experiments, it usually turns out that the energy given by hot water is greater than the energy obtained by cold water. This is explained by the fact that part of the energy is transmitted to the surrounding air, and part of the energy is a vessel in which water was mixed. The equality of the given and the resulting energy will be the more precisely, the less energy losses are allowed in the experience. If you calculate and take into account these losses, the equality will be accurate.
Questions
- What you need to know to calculate the amount of heat obtained by the body when heated?
- Explain the example of how the amount of heat is calculated, the body reported when he heated or released when it is cooling.
- Write the formula to calculate the amount of heat.
- What conclusion can be made from the experience of mixing cold and hot water? Why in practice these energies are not equal?
Exercise 8.
- What amount of heat is required to heat the water with a mass of 0.1 kg per 1 ° C?
- Calculate the amount of heat required for heating: a) cast iron Weighing 1.5 kg to change its temperature by 200 ° C; b) aluminum spoon weighing 50 g from 20 to 90 ° C; c) a brick fireplace weighing 2 tons from 10 to 40 ° C.
- What amount of heat released when cooled water, the volume of which is 20 liters, if the temperature changed from 100 to 50 ° C?
§ 9. Calculation of the amount of heat required for heating the body or the cooling released by it during cooling - physics Grade 8 (PERRIKIN)
Short description:
In paragraph with such a long name, the formula is finally obtained to calculate the amount of heat. All reasoning, conducted in the two previous paragraphs, briefly, in the form of letters denoting physical quantities, are collected in one formula. Values: body weight, changing body temperature, specific heat. This is the first formula in the course of the eighth grade. Sure. After paragraph, nine follows the tasks in which it will be necessary to calculate the amount of heat that is required or stands out. An example of solving such a task is in the textbook. Even two tasks. Specific heatIf it is not specified in the condition of the task, take from the table in paragraph 8.
The amount of heat is associated with the internal energy of the body. If the body gives warmth, the internal energy decreases, and if it receives, then vice versa. Therefore, in tasks, it is sometimes asked to calculate not warmth, but a change in internal energy. So the problem is formulated: "How much did the internal energy changed?" It is necessary to do this by the same formula for the warmth, with which you will get to know this paragraph.

