Why thermal conductivity of liquids is worse than solid bodies. Heat transfer. Types of heat transfer. Thermal conductivity
Thermal conductivity - This is the type of heat transfer, in which the direct transmission of energy from particles (molecules, atoms) is happening to the heated part of the body to particles of its less heated part.
Consider a number of experiments with heating solid, liquid and gas.
Rady heat exchange.
Rady heat exchange - This is a heat exchange, in which energy is transferred to various rays.
It may be sunny rays, as well as rays emitted by heated bodies that are around us.
So, for example, sitting near the campfire, we feel how the heat is transferred from the fire to our body. However, the cause of such a heat transfer cannot be neither thermal conductivity (which in air located between the flame and body is very small), nor convection (since convection flows are always directed up). Here is the third type of heat exchange - rady heat exchange.
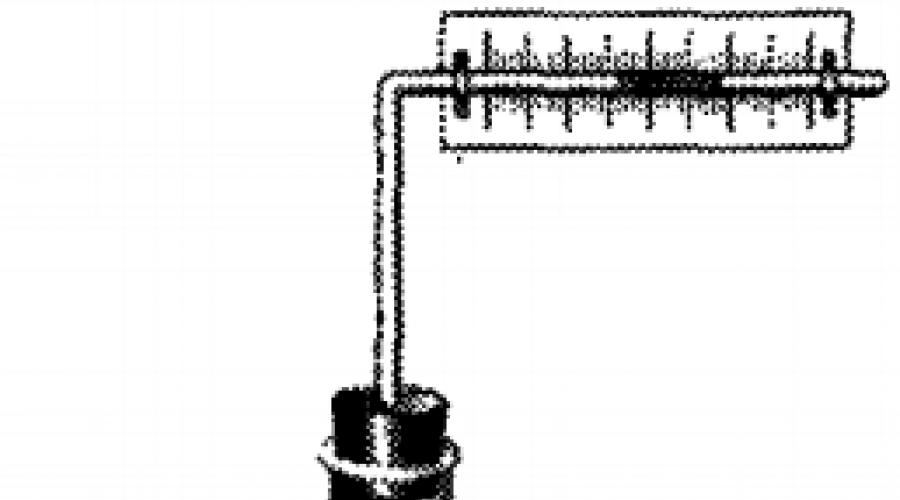
Take a small, wiggy on one side, flask.
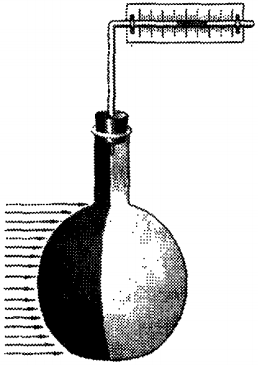
Through the plug into it, the glass tube is bent at the right angle. In this tube, having a narrow channel, we introduce a tinted liquid. Provided on the tube the scale, we get the device - thermoscope. This device allows you to detect even a slight heating of the air in the wrapped flask.
If to bring a piece of metal heated to a high temperature to a dark surface of the thermoscope, then the liquid column will move to the right. Obviously, the air in the flask was heated and expired. The rapid heating of air in the thermoscope can be explained only by transmitting energy from the heated body. As in the case of the fire, the energy here was transmitted not to thermal conductivity and not convective heat exchange. The energy in this case was transmitted using invisible rays emitted by heated body. These rays are called thermal radiation.
The radiant heat exchange can occur in complete vacuum. This is different from other types of heat exchange.
Easily energy all bodies: and strongly heated, and weakly, for example, human body, oven, light bulb. But the higher the body temperature, the stronger its thermal radiation. The emitted energy, reached the outer bodies, is partially absorbed by them, and partially reflected. When absorbing the energy of thermal radiation turns into the inner energy of the bodies, and they are heated.
Light and dark surfaces absorb energy in different ways. So, if in the experiment with a thermoscope, turn the flask to the heated body first, and then with a light side, then the liquid column will move to a greater distance in the first case than in the second (see drawing above). It follows from this that the bodies with a dark surface are better absorbed by energy (and, therefore, heated is stronger) than the bodies with a light or mirror surface.
The bodies with a dark surface are not only better absorb, but also better energies.
The ability to absorb the radiation energy in different ways is widely used in technical. For example, balloons and airplane wings often paint silver paint so that they are less than heated by sunlight.
If you need to use solar energy (for example, for heating some bouts installed on artificial satellites), these devices are painted in a dark color.
1. Introduction.
The project is developed in accordance with the standard of secondary education in physics. When writing this project, the study of heat phenomena is considered, the use of them in everyday life and technique. In addition to theoretical material, much attention is paid research work - These are experiments that answer questions "What methods can be changed in the internal energy of the body", "if the thermal conductivity of various substances", "why the jet of warm air or liquid rise up", "why the bodies with a dark surface are stronger than"; Search and processing of information, photos. Working on the project: 1 - 1.5 months. Project trays: * Practical implementation of knowledge available in schoolchildren about thermal issues; * Formation of skills of independent research activities; * Development of cognitive interests; * Development of logical and technical thinking ; * Development of abilities to the independent acquisition of new knowledge in physics in accordance with life needs and interests;
2. The main part.
2.1. Theoretical part
In life, we really meet with thermal phenomena every day. However, we are not always thinking that these phenomena can be explained if physics is well known. In physics lessons, we became acquainted with the ways of changing internal energy: heat transfer and the performance of work on the body or the body themselves. When contacting two bodies with different temperatures Energy transmission from body with more high temperatures to the body with a lower temperature. This process will occur until the tel temperature is equal to (there will be no thermal equilibrium). In this case, mechanical work is not performed. The process of changing internal energy without working on the body or the body itself is called heat exchange or heat transfer. When heat transfer, the energy is always transmitted from the more heated body to less heated. The reverse process spontaneously (in itself) never happens, that is, the heat exchange is irreversible. The heat exchange determines or accompanies many processes in nature: the evolution of stars and planets, meteorological processes on the ground surface, etc. Types of heat transfer: thermal conductivity, convection, radiation.
Thermal conductivitythe phenomenon of energy transmission from more heated parts of the body is less heated as a result of thermal motion and the interaction of particles from which the body consists of.
Metals have the greatest thermal conductivity - it is hundreds of times more than the water. The exceptions are mercury and lead, but also where thermal conductivity is ten times more than the water.
When lowering the metal knitting needles in a glass with hot water, the end of the knitting needles was very hot. Consequently, internal energy, like any kind of energy, can be transferred from some bodies to others. Internal energy can be transmitted from one part of the body to another. For example, if one end of the nail is heated in the flame, then its other end, which is in hand, gradually heats up and burn hand.
2.2. Practical part.
We study this phenomenon, having done a number of experiments with solid bodies, liquid and gas.
Experience number 1
They took various items: one aluminum spoon, another wooden, third - plastic, fourth - from the stainless alloy, and the fifth - silver. Attached to each spoon with drops of honey paper clips for papers. Invested spoons in a glass with hot water so that the handles with clips stick out from it in different directions. Spoons are warm, and as honey heats up will melt and the clips will disappear.
Of course, spoons should be the same in shape and size. Where heating will happen faster, that metal is better spent heat, better heat. For this experience I took a glass with boiling water and four types of spoons: aluminum, silver, plastic and stainless. I lowered them alone in a glass and kept time: for how many minutes it gets warm. That's what I did:
Conclusion: spoons made of wood and plastic, heat longer than spoons of metal, it means that the metals have good thermal conductivity.
Experience number2
We will enter the end of a wooden stick to the fire. He will ignite. The other end of the stick, which is outside, will be cold. So the tree has bad thermal conductivity.
We bring to the flame of the alcohol end thin glass sticks. After a while he warms up, the other end will remain cold. Consequently, the glass has bad thermal conductivity
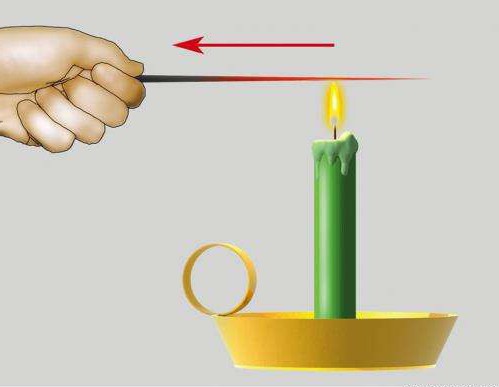
If we heat the end of the metal rod in the flame, then very soon the whole rod is very hot. Hold it in your hands we can no longer.
So, the metals are well carried out heat, i.e. they have a greater thermal conductivity. On the strain-ta-ve-r-zone-tal, but in-crest-lynnogo story. On the rod through one-on-one pro-Major-ki Ver Ti-Cal-But in-crepe-les with a single wax of metallic cloves.
To the edge of the rod-nya under-no-sew candle. Whole-kiland is the edge of the rod-na-g-vas-Xya, then in the art-foal-but-stubbird pro-Ga-Vas-Sia. When the heat is up-to-ho-diet to the place of crepe-lesion of cloves from the rod-it, the ste-a-Rin Plaque, and the carnation pa-gives. We see that in Dan-Mr. experience there is no PE-RE-NO-SA-CA, SO-OT-VET-BUT, NA-BOO-DAYA-SIA Tepl-Lo-Pro-Water .
Experience number 3.
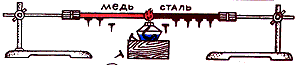
Various metals have different thermal conductivity. In the physical office there is a device with which we can make sure that different metals have different thermal conductivity. However, at home we were able to make sure that the homemade device.
Device for displaying different thermal conductivity of solids.
We made a device for displaying different thermal conductivity. solid tel. To do this, used an empty aluminum foil jar, two rubber rings (homemade), three segments of aluminum, copper and iron wire, tiles, hot water, 3 figures of men with raised up with hands cut out of paper.
The procedure for making the device:
wire bend in the form of the letter "g";
strengthen them on the outside of the bank with the help of rubber rings;
suspend to horizontal parts of wire segments (by means of molten paraffin or plasticine) paper men.
Checking the action of the device. Pour hot water to the jar (if necessary, warm the jar with water on the electric tile) and observe what a figurine falls the first, second, third.
Results. A first figurine falls, fixed on the copper wire, the second - on aluminum, third - on steel.
Output. Different solids have different thermal conductivity.
The thermal conductivity in various substances is different.
Experience number 4.
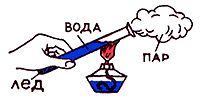
Consider now the thermal conductivity of liquids. Take the test tube with water and get it over top. Water at the surface will soon boil, and at the bottom of the test tube during this time it is just heated. So, fluid thermal conductivity is small.
Experience number 5.
We explore the thermal conductivity of gases. Dry tube put on finger and heat in flame alcohol with a snug up. Finger at the same time will not feel warmth. This is due to the fact that the distance between gas molecules is even greater than that of liquids and solid bodies. Consequently, the thermal conductivity of gases is even less.
Wool, hair, bird feathers, paper, snow and other porous bodies have bad thermal conductivity.
This is due to the fact that there are air between the fibers of these substances. And the air is a bad thermal conductory.
So under the snow, a green grass is saved, wintering are saved from freezing.
Experience number 6.
Fucked a small lump wool and wrapped the thermometer ball to them. And he held a thermometer for some time at a certain distance from the flame and noticed how the temperature rose. Then the same lump wool squeezed and tightly wrapped them the thermometer ball and brought to the lamp again. In the second case, mercury will rise much faster. So, compressed wool carries out heat much better!
The lowest thermal conductivity has a vacuum (space freed from air). It is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs when the interaction of molecules or other particles. In space, where there are no particles, the thermal conductivity cannot be carried out.
3. Conclusion.
In various substances, different thermal conductivity.
Large thermal conductivity has solid bodies (metals), less fluid, and bad - gases.
Thermal conductivity of various substances we can use in everyday life, technique and nature.
The effect of thermal conductivity is inherent in all substances, no matter what aggregate state they are.
Now without difficulty, I can answer and explain from a physical point of view to questions:
1.Why does the birds in cold weather bloom their feathers?
(There is air between feathers, and the air is a bad heat conductor).
2. Why does wool clothes protect better from cold than synthetic?
(Between the fur is air, which is well conducted heat).
3. Why in winter, when the weather is cold, cats sleep, curled into the ball? (Curled into the ball, they reduce the surface area that gives heat).
4. Why do the handles of the soldering iron, irons, pans, make a pan from wood or plastic? (Tree and plastic have bad thermal conductivity, so when heating metal objects, we, holding a wooden or plastic handle, will not burn hands).
5. Why are the bushes of thermal-loving plants and bushes for winter shelter sawdust?
(Sawdust are bad heat conductors. Therefore, plants are covered with sawdusts so that they do not frozen).
6. What boots are better protected from frost: close or spacious?
(Spacious, as the air does not spend heat, it is another layer in the boot, which retains heat).
4. List of references used.
Print editions:
1.A.V. Pryskin Physics Grade 8: Drop, 2012.
2.M.I.Bludov conversation in physics Part1 -m: Education 1984.
Internet resources:
1.Http: //class-fizika.narod.ru/8_3.htm.
2.Http: //ru.wikipedia.org/wiki/%D0%A2%D0%B5%D0%BF%D0%BB%D0%BE%D0%BF%D1%80%D0%BE%D0%B2 % D0% BE% D0% B4% D0% BD% D0% BE% D1% 81% D1% 82% D1% 8C
Heat exchange - This is the process of changing internal energy without working on the body or the body itself.
The heat exchange always occurs in a specific direction: from telly with higher temperatures to bodies with lower.
When the temperature temperatures are aligned, the heat exchange stops.
The heat exchange can be carried out in three ways:
- thermal conductivity
- convection
- radiation
Thermal conductivity
Thermal conductivity - The phenomenon of the transmission of internal energy from one part of the body to another or from one body to another when they are direct contact.
Metals have the greatest thermal conductivity - She's hundreds of times more than the water. Exceptions are mercury and leadBut here thermal conductivity is ten times more than the water.
When lowering the metal knitting needles in a glass with hot water, the end of the knitting needles was very hot. Consequently, internal energy, like any kind of energy, can be transferred from some bodies to others. Internal energy can be transmitted from one part of the body to another. For example, if one end of the nail is heated in the flame, then its other end, which is in hand, gradually heats up and burn hand.
Heating pan on the electric tile occurs through thermal conductivity.
We study this phenomenon, having done a number of experiments with solid bodies, liquid and gas.
We will enter the end of a wooden stick to the fire. He will ignite. The other end of the stick, which is outside, will be cold. It means the tree has bad thermal conductivity.
We bring to the flame of the alcohol end thin glass sticks. After a while he warms up, the other end will remain cold. Consequently, I. glass has bad thermal conductivity.
If we heat the end of the metal rod in the flame, then very soon the whole rod is very hot. Hold it in your hands we can no longer.
It means metals are well carried out heat, i.e. have a greater thermal conductivity. Silver and copper possess the greatest thermal conductivity.
The thermal conductivity in various substances is different.
Wool, hair, bird feathers, paper, plug and other porous bodies have bad thermal conductivity. This is due to the fact that there are air between the fibers of these substances. The lowest thermal conductivity has a vacuum (space freed from air). It is explained by the fact that thermal conductivity is the transfer of energy from one part of the body to another, which occurs when the interaction of molecules or other particles. In space, where there are no particles, the thermal conductivity cannot be carried out.
If there is a need to protect the body from cooling or heating, then apply substances with low thermal conductivity. So, for a saucepan, pan pens from plastic. At home build from logs or bricks with poor thermal conductivity, which means that are protected from cooling.
Convection
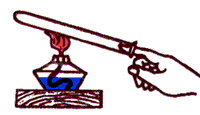
Convection - This is a heat transfer process carried out by transferring the energy to fluid or gas flows.
Example of convection phenomena: A small paper turntable, put over the flame of a candle or a light bulb, under the action of a rising heated air begins to rotate. This phenomenon can be explained in this way. Air, in contact with the warm lamp, heats up, expands and becomes less dense than the cold air surrounding it. Archimedes force acting on warm air from the side of the cold bottom up, more than the power of gravity, which acts on warm air. As a result, the heated air "pops up", rises up, and its place occupies cold air.
When convection, the energy is transferred by the jets of the gas or fluid themselves.
Distinguish two types of convection:
- natural (or free)
- forced
In order for convection in liquids and gases, it is necessary to heat them from below.
Convection in solids can not occur.
Radiation
Radiation - electromagnetic radiation emitted due to the internal energy substance at a certain temperature.
The power of the thermal radiation of the object that satisfies the criteria for absolutely black body is described stephen's law of Boltzmann.
The ratio of radiative and absorption capacity of the bodies is described the law of radiation of Kirchhoff.
Energy transmission by radiation differs from other types of heat transfer: she can be carried out in complete vacuum.
Emit energy all bodies: and strongly heated, and weakly, for example, human body, furnace, electric light bulb, etc. But the higher the body temperature, the more energy it transmits by radiation. In this case, the energy is partially absorbed by these bodies, and partially reflected. When the energy is absorbed, it is heated in different ways, depending on the state of the surface.
Bodies with a dark surface are better absorbed and emitted energy than bodies having a light surface. At the same time, the body with a dark surface is cooled faster by radiation than the bodies with a light surface. For example, in a bright kettle, hot water retains the high temperature than in the dark.
Thermal energy is the term that we use to describe the level of activity of molecules in the object. Increased excitation, one way or another, is associated with an increase in temperature, while in cold objects, atoms are moved much slower.
Examples of heat transfer can be found everywhere - in nature, technique and everyday life.
Examples of heat transfer
The biggest example of heat transfer is the sun that warms the planet the Earth and everything that is located on it. In everyday life, you can find a lot of similar options, only in a much less global sense. So, what examples of heat transfer can be observed in everyday life?
Here is some of them:
Heat is a movement
Thermal flows are in constant motion. The main methods of their transmission can be called the Convention, radiation and conductivity. Let's look at these concepts in more detail.
What is conductivity?
Perhaps many more than once noticed that in the same room the sensation of touch with the floor can be completely different. It's nice and warm to walk around the carpet, but if you go to the bathroom with barefoot legs, tangible coolness immediately gives a feeling of vigor. Only not in the case where there is heated floors.

So why is the tile surface frozen? This is all due to thermal conductivity. This is one of three types of heat transfer. Whenever two objects of different temperatures are in contact with each other, the thermal energy will be held between them. Examples of heat transfer in this case can be given as follows: Holding for a metal plate, the other end of which will be placed above the candle flame, and over time you can feel burning and pain, and at the time of touching the iron handle, boiling water can be obtained.
Conduction factors
Good or bad conduction depends on several factors:
- The view and quality of the material from which the items are made.
- The surface area of \u200b\u200btwo objects in contact.
- The temperature difference between two objects.
- Thickness and size of objects.

In the form of the equation, it looks like this: heat transfer rate to the object is equal to the thermal conductivity of the material from which the object is made multiplied by the surface area in contact multiplied by the temperature difference between two objects and divided into the thickness of the material. Everything is simple.
Examples of conductivity
Direct heat transfer from one object to another is called conduction, and substances that are warmly conducted are called conductors. Some materials and substances do not cope with this task, they are called insulators. These include wood, plastic, fiberglass and even air. As you know, the insulators actually do not stop the heat flow, but simply slow down to one degree or another.
Convection
This type of heat transfer as convection occurs in all fluids and gases. You can meet such examples of heat transfer in nature and in everyday life. When the liquid heats up, the molecules at the bottom are gaining energy and start moving faster, which leads to a decrease in density. Warm fluid molecules start moving up, while the cooler (more dense fluid) begins to sink. After the cool molecules reach the bottom, they again get their share of energy and again seek to the top. The cycle continues as long as there is a heat source at the bottom.
Examples of heat transfer in nature can be given as follows: With a special equipped burner, warm air, filling the space of the balloon, can raise the entire design to a sufficiently large height, the whole thing is that warm air is lighter than cold.
Radiation
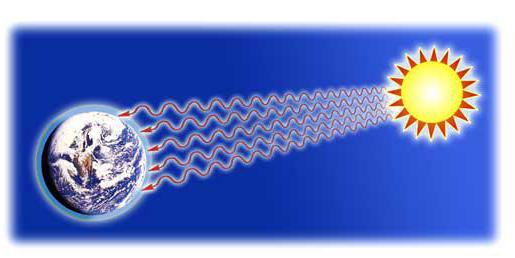
When you sit in front of the fire, you warm the heat outgoing from it. The same thing happens if you bring the palm to the burning light, without touching it. You will feel warm too. The largest examples of heat transfer in everyday life and nature heads solar energy. Every day the heat of the sun passes through 146 million km of empty space until the earth itself. This is a driving force for all forms and systems of life that exist on our planet today. Without this method of transmission, we would be in big trouble, and the world would not be at all how we know it.

Radiation is heat transfer using electromagnetic waves, be it radio wave, infrared, x-rays or even visible light. All objects emit and absorb radiant energy, including the person himself, but not all objects and substances should cope with this task in the same way well. Examples of heat transfer in everyday life can be considered using a conventional antenna. As a rule, what radiates well is also good and absorbs. As for the land, it takes energy from the Sun, and then gives it back into space. This radiation energy is called earthly radiation, and this is what makes a possible life in the planet possible.
Examples of heat transfer in nature, everyday life, technician
Energy transmission, in particular, thermal, is a fundamental area of \u200b\u200bresearch for all engineers. Radiation makes land suitable for habitat and gives renewable solar energy. Convection is the basis of mechanics, is responsible for air flows in buildings and air exchange in houses. The conductivity allows you to heat the pan, just putting it on fire.
Numerous examples of heat transfer in the art and nature are obvious and occur everywhere in our world. Almost all of them play a big role, especially in the field of mechanical engineering. For example, when designing a system of ventilation of the building, engineers calculate the heat transfer of the building in its surroundings, as well as the internal transmission of heat. In addition, they choose materials that minimize or maximize heat transmission through separate components to optimize efficiency.
Evaporation
When the fluid atoms or molecules (for example, water) are exposed to a significant amount of gas, they tend to spontaneously enter the gaseous state or evaporate. This is because the molecules are constantly moving in different directions at random speeds and face each other. During these processes, some of them get kinetic energy sufficient to repel from the heating source.

However, not all molecules have time to evaporate and become a water vapor. It all depends on temperature. So, the water in the glass will evaporate more slowly than in the stew heated on the stove. Boiling water significantly increases the energy of molecules, which, in turn, speeds up the process of evaporation.
Basic concepts
- Conductivity is heat transmission through a substance with direct contact of atoms or molecules.
- Convection is heat transfer due to gas circulation (for example, air) or liquid (for example, water).
- Radiation is the difference between the absorbed and reflected amount of heat. This ability strongly depends on the color, black objects absorb more heat than light.
- Evaporation is a process in which atoms or molecules in liquid state are produced sufficiently energy to become gas or steam.
- - These are gases that delay the heat of the sun in the earth atmosphere, producing a greenhouse effect. Allocate two main categories - it is water vapor and carbon dioxide.
- - These are infinite resources that are quickly and naturally replenished. This includes the following examples of heat transfer in nature and technology: winds and the energy of the Sun.
- The thermal conductivity is the speed with which the material transmits thermal energy through itself.
- The thermal equilibrium is a state in which all parts of the system are in the same temperature mode.
Application in practice
Numerous examples of heat transfer in nature and technology (pictures above) indicate that these processes should be well studied and served in good. Engineers apply their knowledge of the principles of heat transfer, are investigating new technologies that are related to the use of renewable resources and are less destructive to the environment. Key Moment It is an understanding that energy transfer opens endless opportunities for engineering solutions and not only.
The heat transfer is one of the ways to change the inner energy of the body (or system of tel), while the internal energy of one body goes into the internal energy of another body without performing mechanical work.
There are 3 types of heat transfer:
The heat exchange between the two media occurs through the solid wall separating them or through the surface of the section between them.
The heat is able to move only from the body with a higher temperature to the body less heated.
The heat exchange always flows so that the loss of the internal energy of some bodies is always accompanied by the same increment to the internal energy of other bodies involved in heat exchange.
This is a special case of the law of energy conservation.
INTERESTING
Partridges, ducks and other birds are not frown in winter because they may differ from the body temperature by more than 30 degrees. Low papal temperature strongly lowers heat transfer. These are the protective forces of the body!
The thermal conductivity is the transfer of energy from more heated areas of the body to less heated due to thermal motion and the interaction of microparticles (atoms, molecules, ions, etc.), which leads to leveling body temperature.
Not accompanied by the transfer of the substance!
This type of internal energy transmission is characteristic of both solids and liquids and gases.
The thermal conductivity of various substances is different.
Metals have the highest thermal conductivity,

moreover, different metals thermal conductivity differ.
Liquids have less thermal conductivity than solid bodies, and gases are smaller than liquids.
When the upper end is heated with a finger-closed test tube with air inside, you can not be afraid to burn your finger, because Thermal conductivity of gases is very low.
It is interesting that you could bring your hand almost close to the flame, for example, the gas burner (the temperature is more than 1000 degrees) and do not burn it if ...
And what if?

Gas, as a rule, a very bad heat conductor, therefore it would be enough for only a small air layer between hand and flame. But!
But there is a phenomenon as convection in gases, so near the flame hand burns greatly.
Look at the bookshelf
Do you know that ...

Big difficulties builder builders give the foundation drawdown especially in the regions with permafrost. Houses often give cracks due to the fitting of the soil under them the foundation transfers the soil some amount of heat. Therefore, the buildings began to build on piles. In this case, heat is transmitted only with thermal conductivity from the foundation of the pile and further from the pile soil from what should be done by piles? It turns out that piles made of durable solid material inside should be filled with kerosene. In summer, the pile is heated from top to bottom, because Liquid has low thermal conductivity. In winter, it falling down due to the convection of the fluid inside it, on the contrary, will contribute to the additional cooled of the soil.
This is not a fairy tale, not fiction!
Such a project is really designed and tested!
Italian scientists invented a shirt that allows maintenance of a constant body temperature. Scientists promise that in the summer it will not be hot in it, and in winter it is cold, because it is sewn from special materials. Such materials are already used in space flights.
In the old machine guns "Maxim" heating water prevented a weapon from melting.
In the kitchen, raising the dishes filled with hot liquid, so as not to burn, you can only use a dry rag. The thermal conductivity of the air is much less than the water! And the fabric structure is very loose, and all the precasts between the fibers are filled with a dry rag with air, and in wet water. Look, do not burn!
Fire in Serete

The phenomenon about which is described below demonstrates the properties of the metals well to carry out heat.
If we make a wire mesh, providing a good metal connection in places crossing the wire, and put it above the gas burner, then you can set up the gas on the valve on the valve, while under the grid it will not burn. And if you light gas under the grid, then the fire "does not leak" over the grid!
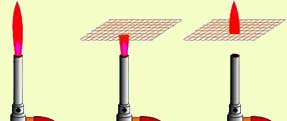
In those days, when there were no electric mining light bulbs, they used Davy lamp.
It was a candle, "planted" into the metal cage. And even if the mine was filled with flammable gases, the Davy lamp was safe and did not cause an explosion - the flame did not go beyond the lamp due to the metal grid.
